piCHEM provides support for all phases of drug development
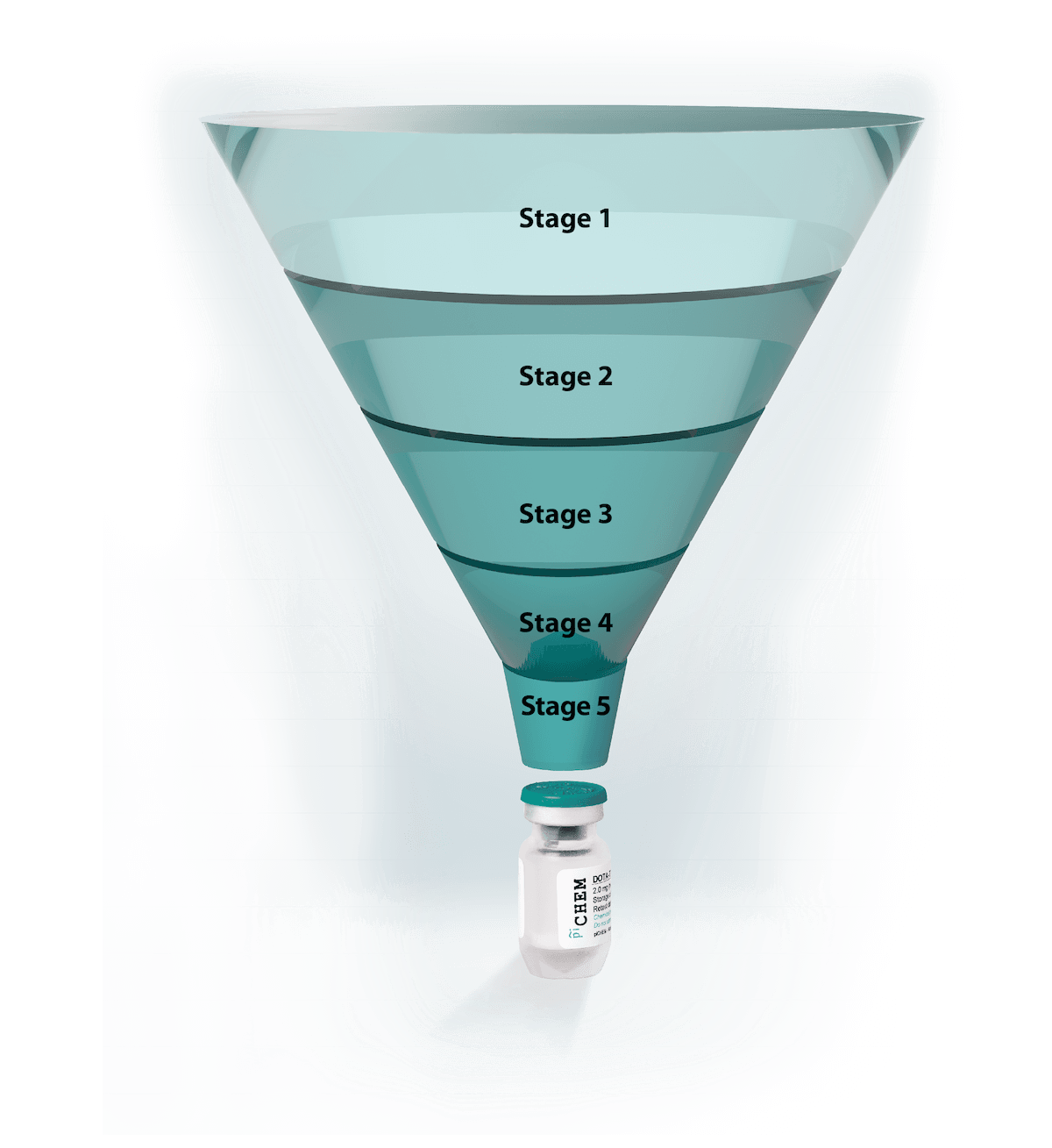
Stage 1 – Basic Research
- R&D custom peptide synthesis
- Peptide structure design
- Synthesis strategy
- Modification
- Dye labeling
- Peptidomimetic
- PNAs
- Flexible from milligram to multi-gram scale
Stage 2 – Pre-clinical Development
- Toxicology lots – Technical lots
- Initial process development
- Analytical method development
- Formulation studies
- Container compatibility studies
Stage 3 – Clinical Stage – Phase I to III
- EU-GMP/cGMP production
- Process scale-up
- Gram to several hundred grams
- Process validation
- Analytical method development
- Analytical method validation
- Stability studies
- GMP documentation
- QP release
- Formulation development
- Aseptic fill & finish service
- Manufacture of reference material
Stage 4 – Regulatory Affairs
- Preparation of quality documentation IND/IMPD
- Preparation of DMF/ASMF
- Submission of documents to authorities
- Dossier lifecycle management
Stage 5 – Commercial Manufacturing
- Production according to marketing authorisation
- Contract manufacturing
- QP release
- Supply management
Over the last 30 years piCHEM worked within several EU co-financed collaborative projects and has become an experienced and reliable partner for research projects.